

An alloy of chromium, cobalt, and nickel has just given us the highest fracture toughness ever measured in a material on Earth.
It has exceptionally high strength and ductility, leading to what a team of scientists has called "outstanding damage tolerance".
Moreover – and counterintuitively – these properties increase as the material gets colder, suggesting some interesting potential for applications in extreme cryogenic environments.
"When you design structural materials, you want them to be strong but also ductile and resistant to fracture," says metallurgist Easo George, Governor's Chair for Advanced Alloy Theory and Development at Oak Ridge National Laboratory and the University of Tennessee.
"Typically, it's a compromise between these properties. But this material is both, and instead of becoming brittle at low temperatures, it gets tougher."
Strength, ductility, and toughness are three properties that determine how durable a material is. Strength describes resistance to deformation. And ductility describes how malleable a material is. These two properties contribute to its overall toughness: the resistance to fracture. Fracture toughness is the resistance to further fracture in an already-fractured material.
George and fellow senior author, mechanical engineer Robert Richie of Berkeley National Laboratory and the University of California, Berkeley, have spent some time working on a class of materials known as high-entropy alloys, or HEAs. Most alloys are dominated by one element, with small proportions of others mixed in. HEAs contain elements mixed in equal proportions.
One such alloy, CrMnFeCoNi (chromium, manganese, iron, cobalt, and nickel), has been the subject of intense study after scientists noticed that its strength and ductility increase at liquid nitrogen temperature without compromising toughness.
One derivative of this alloy, CrCoNi (chromium, cobalt, and nickel), displayed even more exceptional properties. So George and Ritchie and their team cracked their knuckles and set about pushing it to its limits.
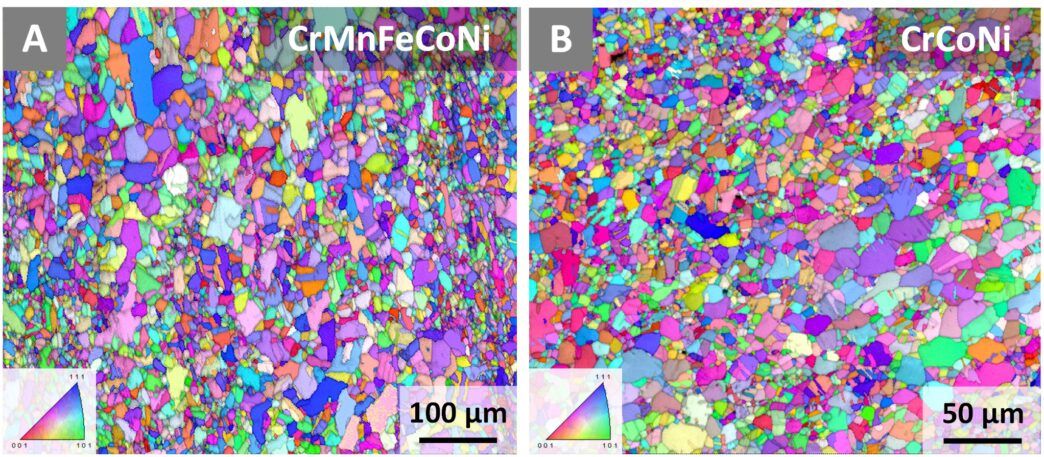
The previous experiments on CrMnFeCoNi and CrCoNi had been conducted at liquid nitrogen temperatures, up to 77 Kelvin (-196°C, -321°F). The team pushed it even further, to liquid helium temperatures.
The results were beyond striking.
"The toughness of this material near liquid helium temperatures (20 Kelvin, [-253°C, -424°F]) is as high as 500 megapascals square root meters," Ritchie explains.
"In the same units, the toughness of a piece of silicon is one, the aluminum airframe in passenger airplanes is about 35, and the toughness of some of the best steels is around 100. So, 500, it's a staggering number."
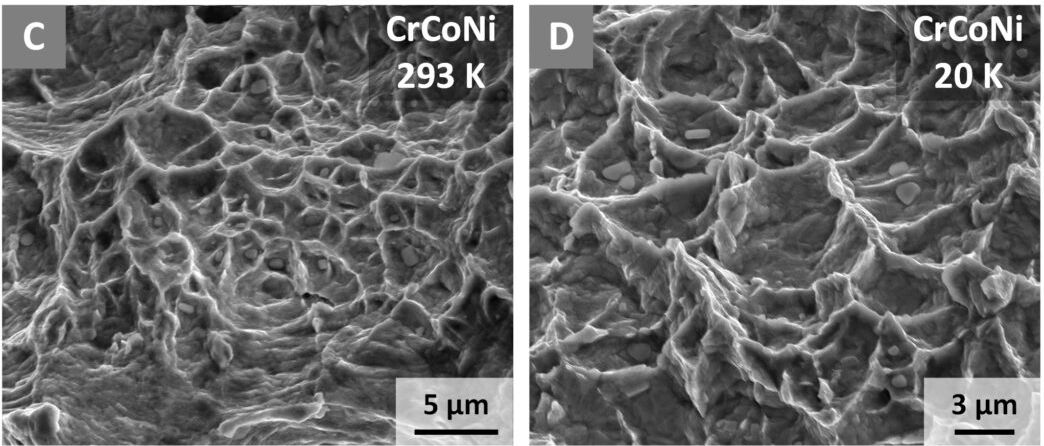
To figure out how it works, the team used neutron diffraction, electron backscatter diffraction, and transmission electron microscopy to study CrCoNi down to the atomic level when fractured at room temperature and in extreme cold.
This involved cracking the material and measuring the stress required to cause the fracture to grow and then looking at the crystalline structure of the samples.
Atoms in metals are arranged in a repeating pattern in three-dimensional space. This pattern is known as the crystal lattice. The repeating components in the lattice are known as unit cells.
Sometimes boundaries are created between unit cells that are deformed and those that aren't. These boundaries are called dislocations, and when force is applied to the metal, they move, allowing the metal to change shape. The more dislocations a metal has, the more malleable it is.
Irregularities in the metal can block the dislocations from moving; this is what makes a material strong. But if the dislocations are blocked, instead of deforming, a material can crack, so high strength can often mean high brittleness. In CrCoNi, the researchers identified a particular sequence of three dislocation blocks.
The first to occur is slip, which is when parallel parts of the crystal lattice slide away from each other. This causes the unit cells to no longer match up perpendicular to the slip direction.
Continued force produces nanotwinning, where crystal lattices form a mirrored arrangement on either side of a boundary. If yet more force is applied, that energy goes into rearranging the shape of the unit cells, from a cubic to a hexagonal crystal lattice.
"As you are pulling it, the first mechanism starts, and then the second one starts, and then the third one starts, and then the fourth," Ritchie says.
"Now, a lot of people will say, well, we've seen nanotwinning in regular materials, we've seen slip in regular materials. That's true. There's nothing new about that, but it's the fact they all occur in this magical sequence that gives us these really tremendous properties."
The researchers also tested CrMnFeCoNi at liquid helium temperatures, but it did not perform nearly as well as its simpler derivative.
The next step will be to investigate the potential applications of such a material, as well as finding other HEAs with similar properties.
The research has been published in Science.









 Add Category
Add Category