
Some artificial tears, or eye drops for dry eyes, are linked to a multistate investigation into a cluster of infections that have resulted in vision loss, hospitalization and one death, according to a Wednesday alert for health care providers from the US Centers for Disease Control and Prevention.
On Thursday, the US Food and Drug Administration posted a voluntary recall by Global Pharma Healthcare including two brands: EzriCare's and Delsam Pharma's artificial tears lubricant eye drops. The eye drops could be contaminated, according to the recall, and may be associated with the CDC's ongoing investigation into "extensively drug-resistant" infections that weren't responsive to a range of antibiotics.
Most patients with infections included in the CDC's investigation reported using artificial tears. Cases date back to May 2022, with 55 cases in 12 states. Many were linked to four different health care facilities. The most common brand name reported was EzriCare, prompting the CDC to recommend people stop using that brand for the time being before a recall was posted by the FDA.
However, patients reported using over 10 brands of artificial tears, according to the alert, sometimes using multiple brands. Global Pharma Healthcare manufactures both EzriCare and Delsam Pharma drops -- the only brands currently included in the recall.
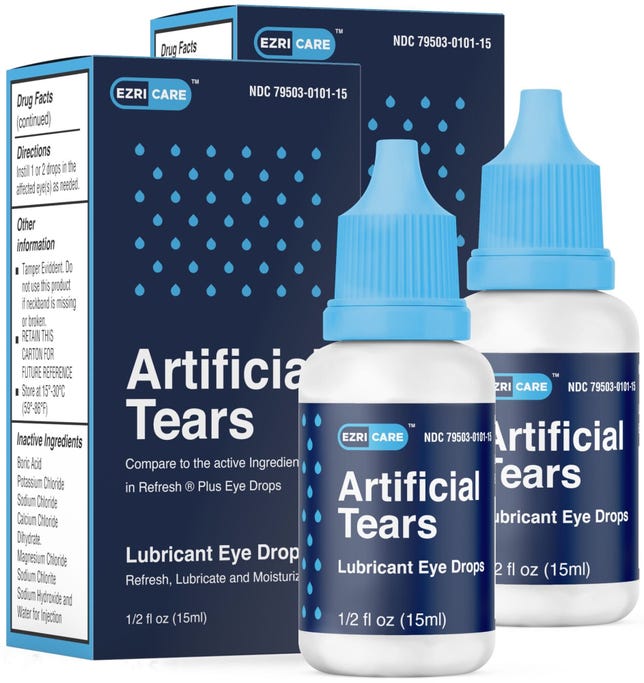
EzriCare said Wednesday that customers should discontinue use of its lubricating eye drops, and that as soon as the company received word of the CDC's investigation notice, EzriCare "immediately took action to stop any further distribution or sale." The company also said it's willing to work with health agencies, but it added that it's "not aware of any testing that definitively links" the infections to EzriCare's Artificial Tears.
In a statement, Global Pharma Healthcare said it's "fully cooperating" with health authorities in the US in the investigation, but so far "we have not determined whether our manufacturing facility is the source of the contamination."
"Nevertheless, out of an abundance of caution, we are recalling the products at issue," the manufacturer told CNET.
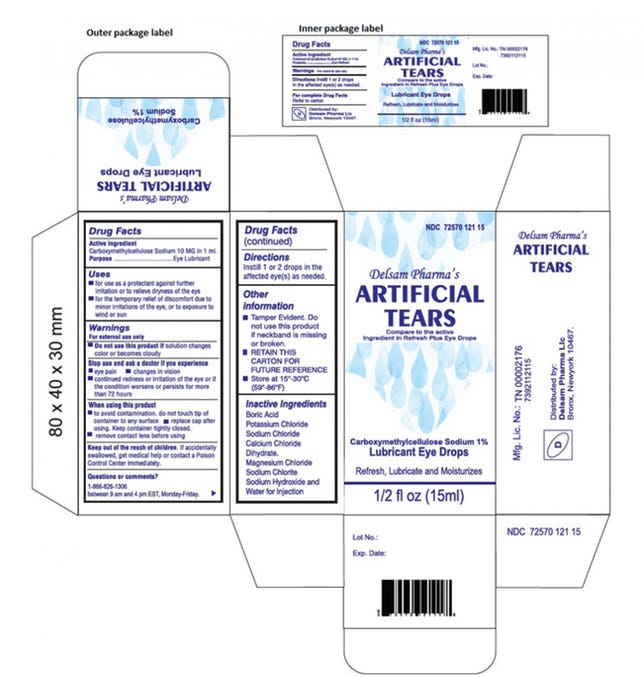
Delsam Pharma, the other eye drop brand manufactured by Global Pharma Healthcare and included in the recall, said that the company is following the recall request. Delsam also noted that the CDC hasn't linked Delsam's eye drops to its investigation. Its drops are part of the recall because they're manufactured by the same company that makes EzriCare's.
Artificial tears are often used for treating dry eyes. Preservative-free ones like the ones being recalled have fewer additives that are meant to discourage bacteria growth. However, they might be recommended for people who use them frequently or multiple times a day.
Symptoms of an eye infection include discharge from the eye (yellow, green or clear), eye pain, redness of the eye or eyelid, feeling like something's in your eye, increased sensitivity to light and blurry vision, according to the CDC. Eye infections can be serious and you should seek medical care right away if you have symptoms.
The current artificial tear recall affects all lots "within expiry," of both brands. If you have questions, you can contact EzriCare's distributor at 1-518-738-7602 or arupharmainc@yahoo.com, and Delsam Pharma at 1-866-826-1309 or delsampharma@yahoo.com.
The information contained in this article is for educational and informational purposes only and is not intended as health or medical advice. Always consult a physician or other qualified health provider regarding any questions you may have about a medical condition or health objectives.









 Add Category
Add Category